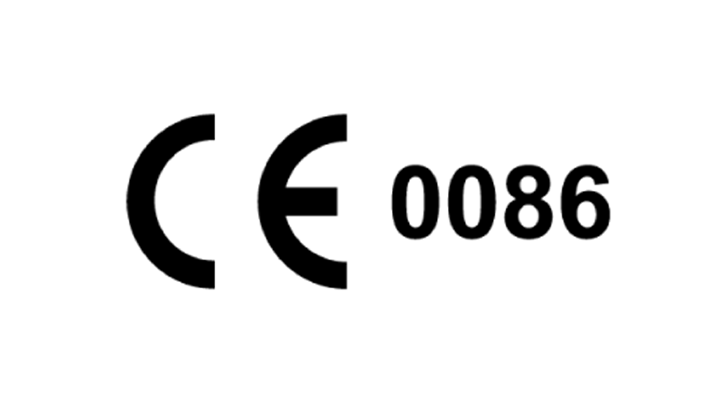
Characterization of suspected dermal fillers containing hyaluronic acid - Analytical Methods (RSC Publishing) DOI:10.1039/C7AY01130J
Organismos Notificados MDR (25): SGS Belgium (Belgica) ON num. 1639 nuevo ON. Enhorabuena @SGS_Spain !!!

CGM accuracy: Contrasting CE marking with the governmental controls of the USA (FDA) and Australia (TGA): A narrative review - Pemberton - 2023 - Diabetes, Obesity and Metabolism - Wiley Online Library

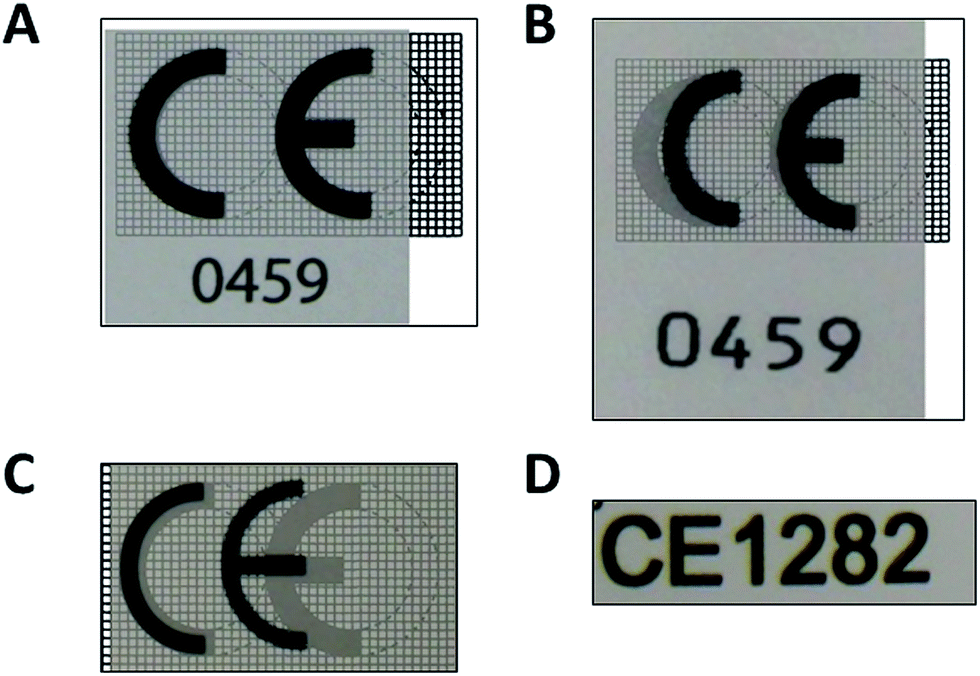
Organismos Notificados: GMED (Francia) NB num. 0459 nuevo ON (ya 15) con MDR. Enhorabuena!!! | Red de Tecnologías Sanitarias y Productos Sanitarios

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

Organismos Notificados: UDEM Adriatic d.o.o. (Croacia) ON num. 2696 nuevo ON (ya 18) con MDR. Enhorabuena!!! | Red de Tecnologías Sanitarias y Productos Sanitarios

Neuronavigation, in Vitro Diagnostics, European Economic Community, transcranial Magnetic Stimulation, ISO 13485, cE Marking, european Commission, directive, medical Device, Regulation | Anyrgb


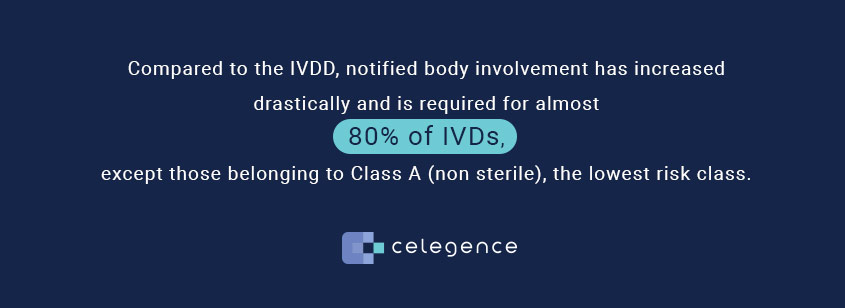



.jpg)