1.1.7 Ionisation Energy: Trends & Evidence | AQA A Level Chemistry Revision Notes 2017 | Save My Exams

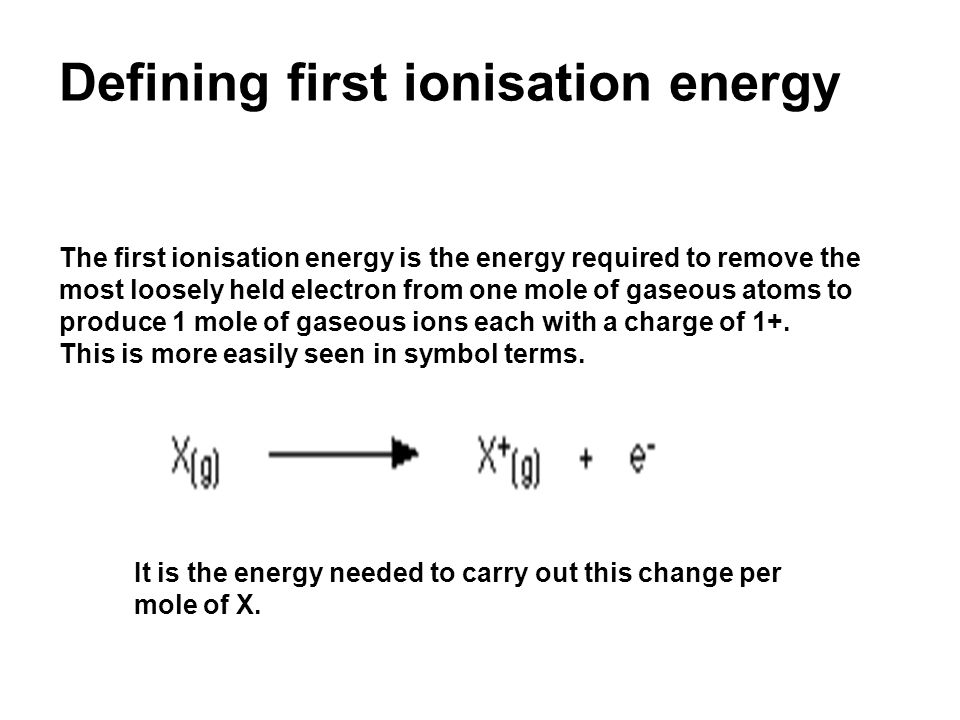
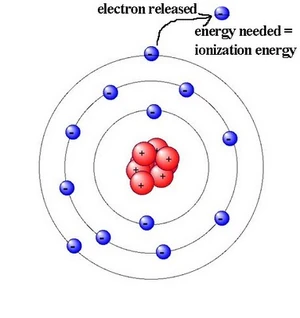
PPT - Lesson objectives • Define first ionisation energy and successive ionisation energy. PowerPoint Presentation - ID:5937155
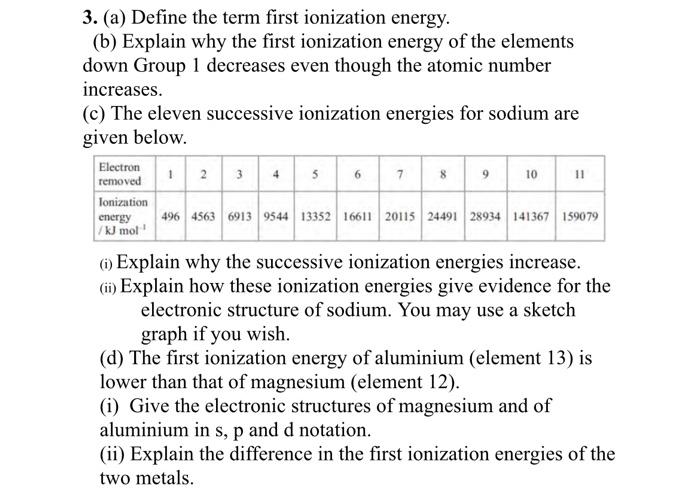
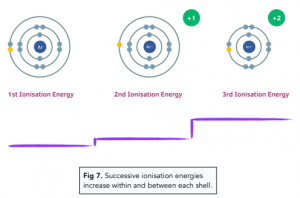
The first, second and third ionisation energies of Al are 578, 1817 and 2745 kJ mol^ 1 respectively. Calculate the energy required to convert all the atoms of Al to Al^+3 present

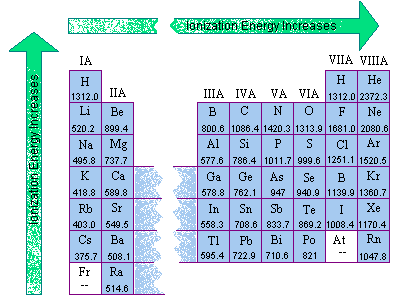
Difference Between First and Second Ionization Energy (I1E vs I2E) | Compare the Difference Between Similar Terms

Lesson objectives Define first ionisation energy and successive ionisation energy. Explain the factors that influence ionisation energies. Predict the. - ppt download

PPT - Learning Objectives: •Define first ionisation energy and successive ionisation energy. PowerPoint Presentation - ID:2354952