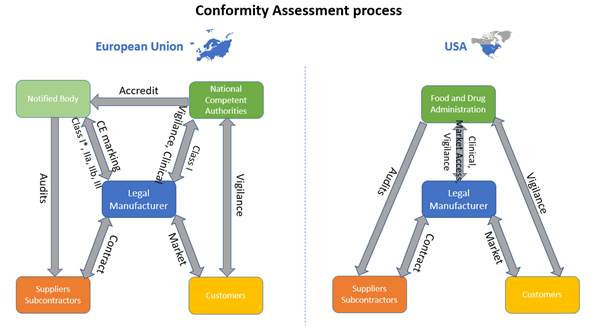
What are the principal differences between the conformity assessment process of a medical device in the USA and in the European Union? - Kvalito
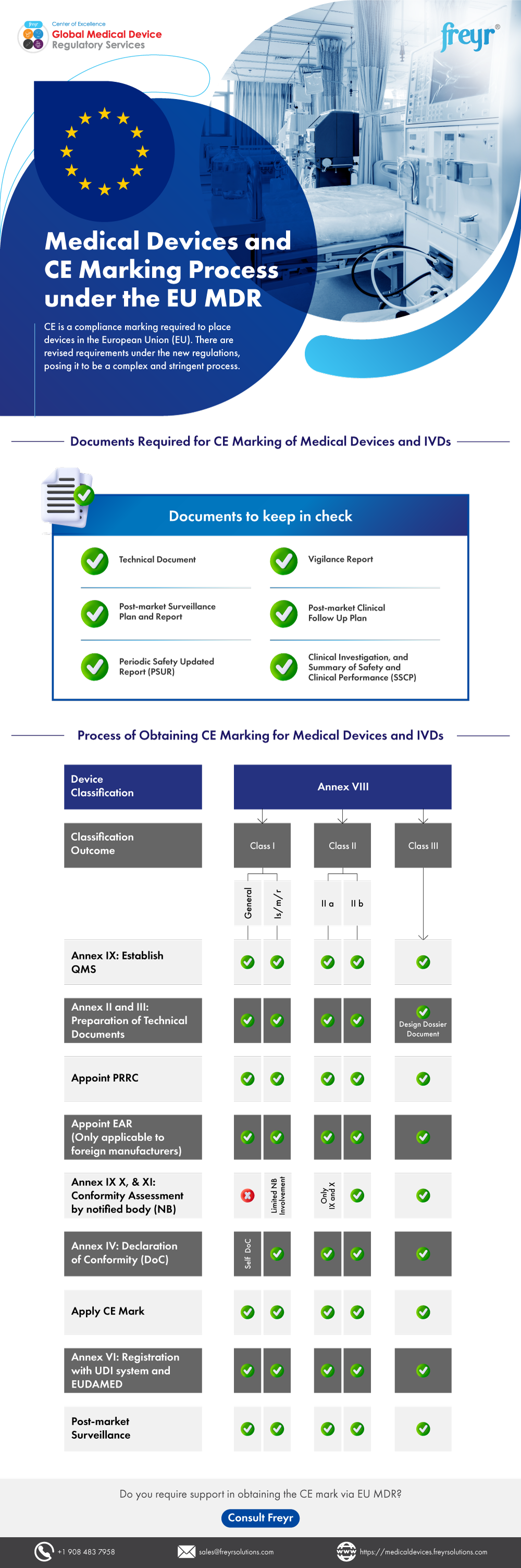
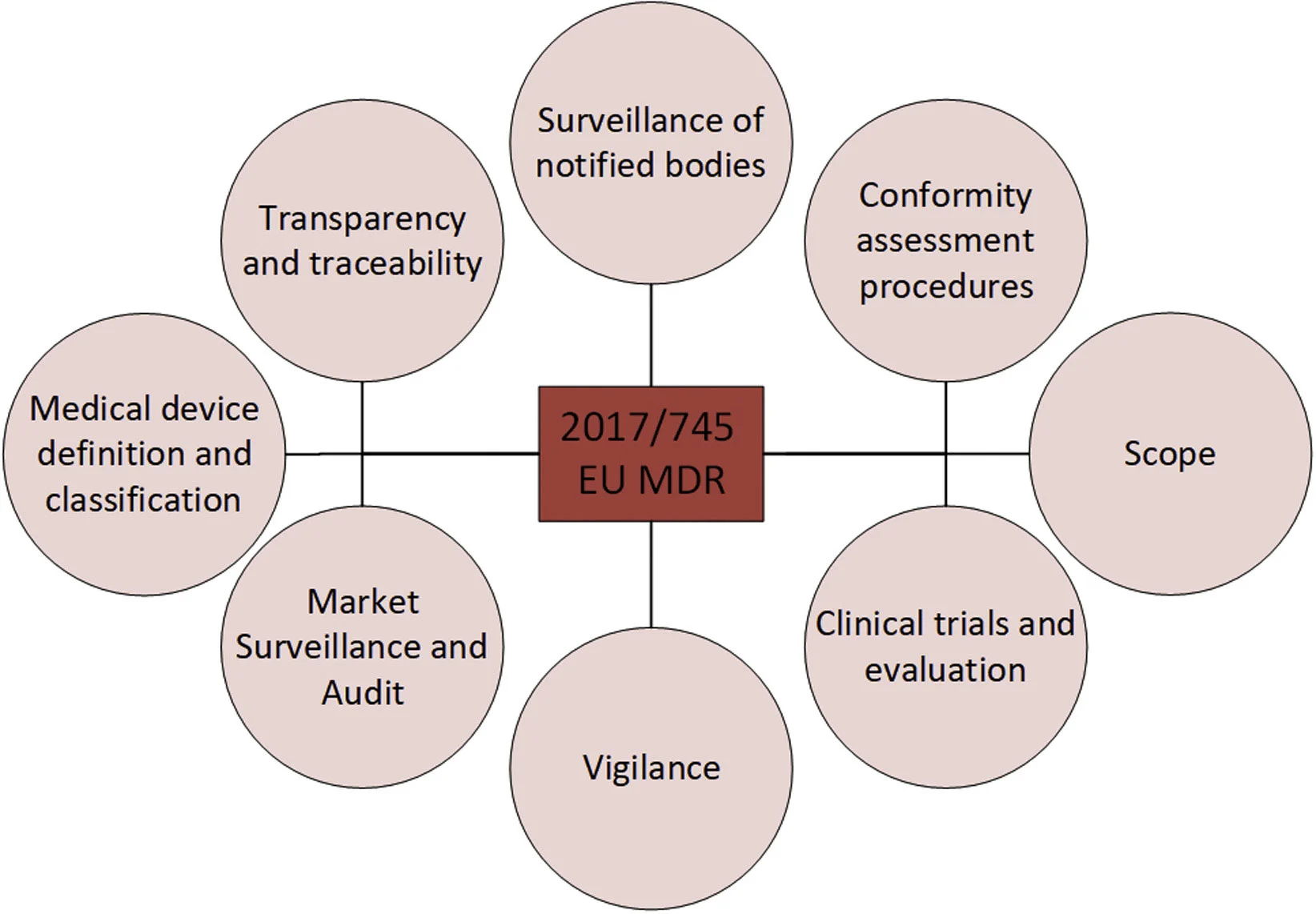
Medical Devices and CE Marking Process under the EU MDR | Freyr - Global Regulatory Solutions and Services Company

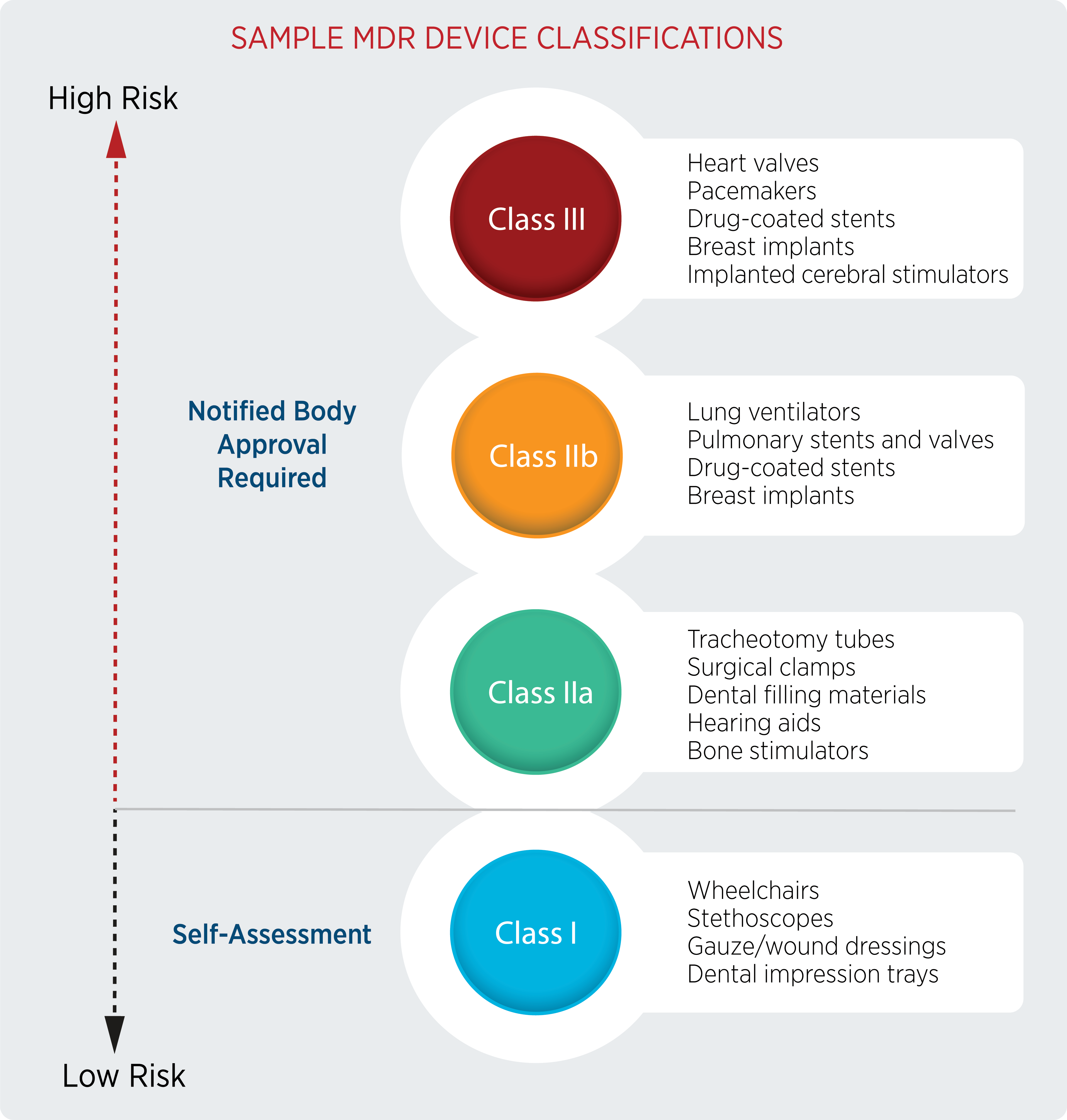
EU Finalizes New Medical Device Regulations (MDR) which update the regulatory framework for the marketing of devices and IVDs in Europe – Catchtrial

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

What is the lifetime of my medical device? Learn from Team-NB's position paper. | Beat Keller posted on the topic | LinkedIn


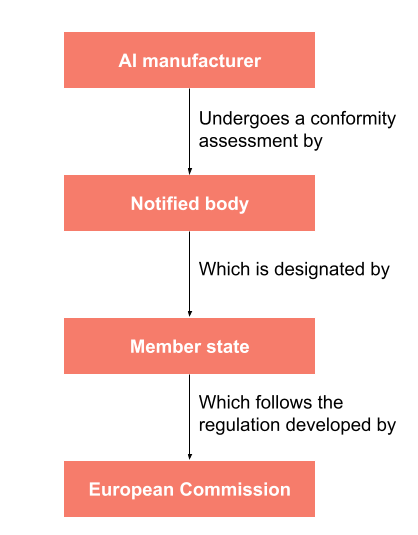
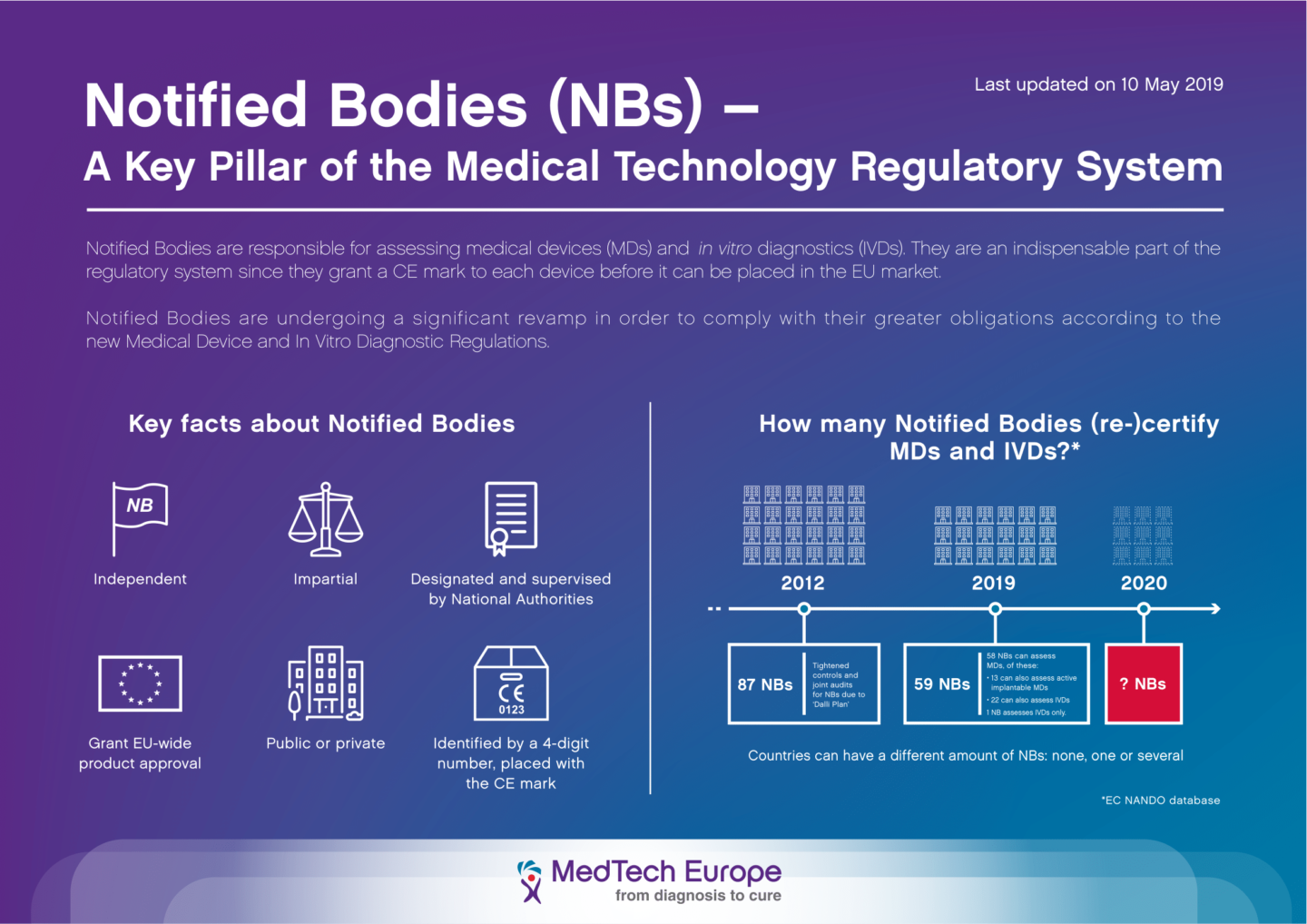

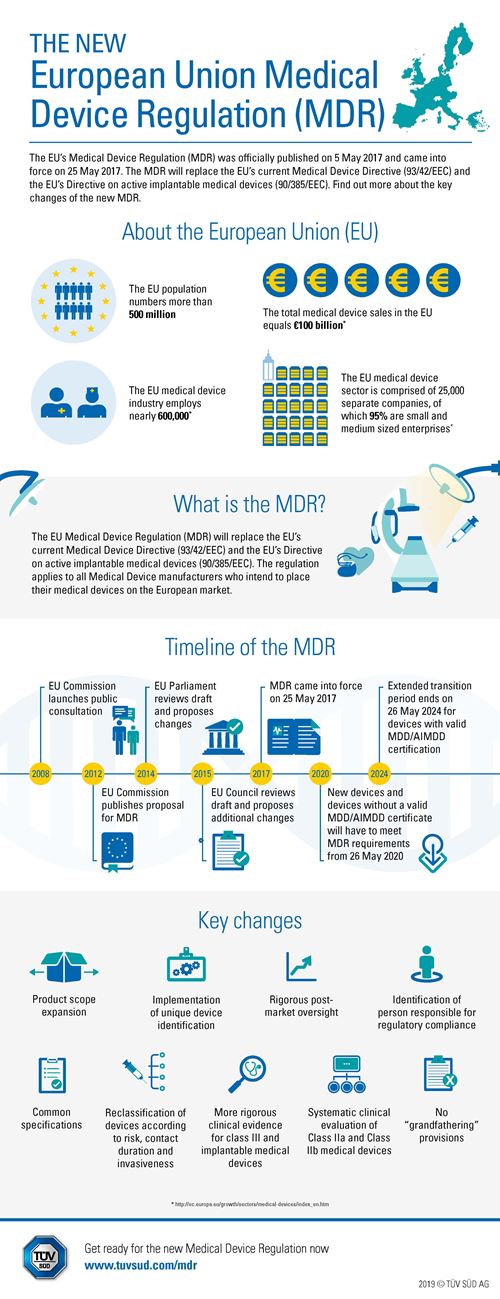



/tuv-rheinland-ivdr-visual-1-en.png)